MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:

MLZ (eng)
Lichtenbergstr.1
85748 Garching
15.07.2016
Neutrons observe the effects of Alzheimer’s peptides
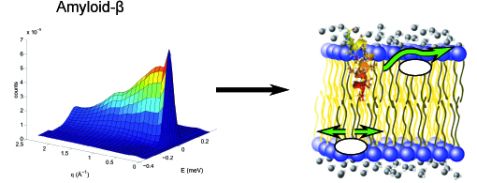
The dynamics of the lipids in the membrane change significantly, with the presence of the Alzheimer peptide amyloid-β. (Left: Exemplary result from a ToFToF measurement. Right: A model of the lipid dynamic behaviour right). © M. Barrett (HZB)
Using neutrons, scientists have revealed a possible cause of Alzheimer’s disease. During recent experiments carried out on the instrument TOFTOF at Heinz Maier-Leibnitz Zentrum in Garching and two spectrometers at the ILL in Grenoble, they observed an altered mobility of the lipids in model cell membranes with the presence of amyloid-β. This peptide is believed to trigger symptoms of Alzheimer’s disease.
In a brain affected by Alzheimer’s disease, plaques are observed outside of the nerve cells. These plaques consist predominantly of the peptide amyloid-β. The neurotoxic effect leading to the symptoms of Alzheimer’s disease is also attributed to this peptide which is active in the membranes of nerve cells individually or in small aggregates. However, the method in which amyloid-β kills the nerve cells is still controversial. Studies suggest that the peptides penetrate the lipid layers of membranes and form pores making the cells permeable, porous and thus damaged.
Dr. Matthew Barrett of the Helmholtz Zentrum Berlin and his colleagues used neutron scattering to investigate the effect amyloid-β has on cell membranes, comparing the dynamics of membranes with and without the peptide present. They used the time-of-flight spectrometer TOFTOF operated by Dr. Wiebke Lohstroh of the Technical University of Munich in Garching and two other neutron spectrometers at the Institut Laue-Langevin in Grenoble, France. TOFTOF enabled the investigation of the movement and diffusion of lipids in the membrane on a time scale of 1 – 100 picoseconds and on length scales of a few nanometers, while the measurements at the spectrometers at the ILL probed slower motions.
The biophysicists observed that small amounts of amyloid-β significantly influence the movements of the lipids in the membrane. The authors suggest that the altered mobility could be even more harmful to the normal function of the cell membrane than pore formation, which is often associated with the peptide. Signal transmittance might be impeded and prohibit the brain from carrying out normal functions, eventually leading to cell death. The scientists hypothesize that the plaques of amyloid-β which occur in connection with Alzheimer’s disease may a kind of trashbin for the harmful peptide: the stray amyloid-β peptides are collected in the plaques, thereby protecting the cell membranes from lone peptides.
Original publication:
Alzheimer’s peptide amyloid-β, fragment 22-40, perturbs lipid dynamics
Barrett MA, Trapp M, Lohstroh W, Seydel T, Ollivier J, Ballauff M, Dencher NA, Hauß T.
Soft Matter. 2016 Feb 7; 12(5):1444-51,
doi: 10.1039/c5sm02026c
MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:



